Airway smooth muscle (ASM) hypercontractility – another cause of airway hyperresponsiveness?
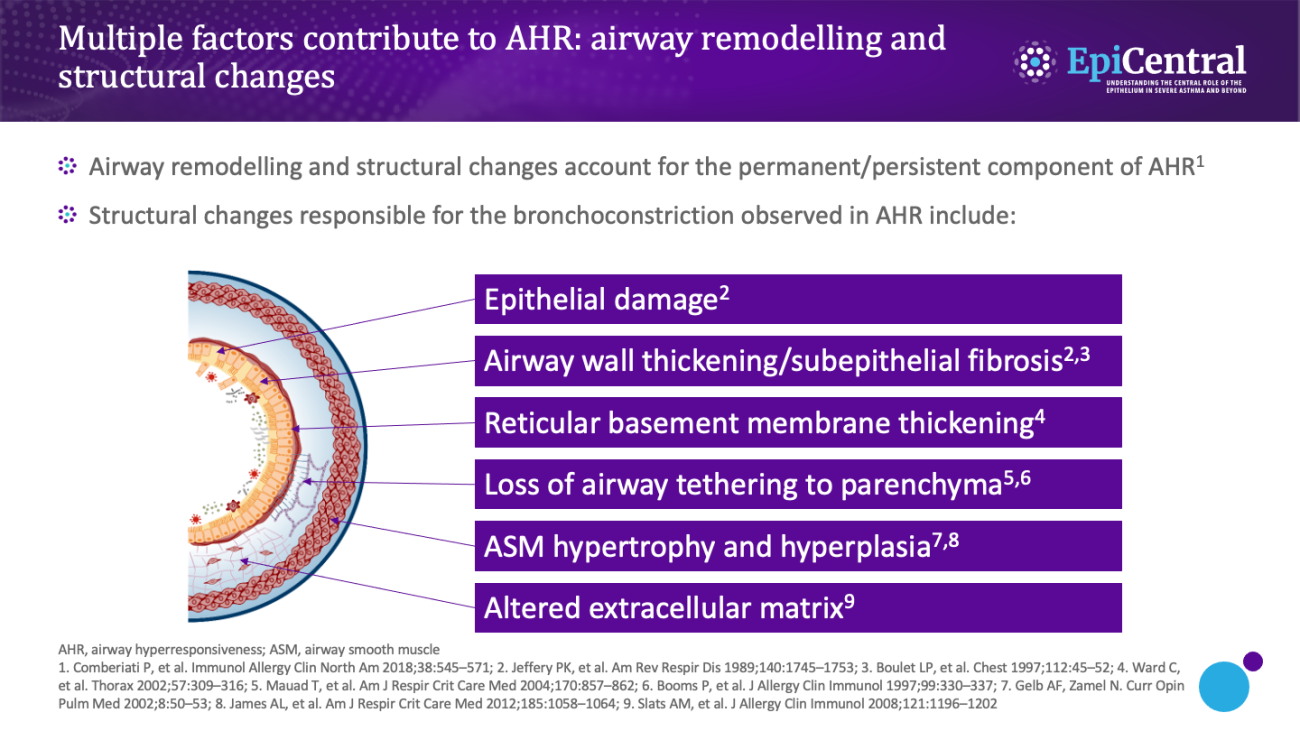
Distinct from the structural changes attributed to airway remodelling, in-vitro studies have shown that patients with asthma exhibit fundamental physiological changes in their ASM.2,12 Compared with ASM from healthy individuals, ASM from patients with asthma displays enhanced shortening and increased contractility, which is hypothesised to be another cause of airway hyperresponsiveness.2,12
What is airway inflammation, and how are mast cells involved?
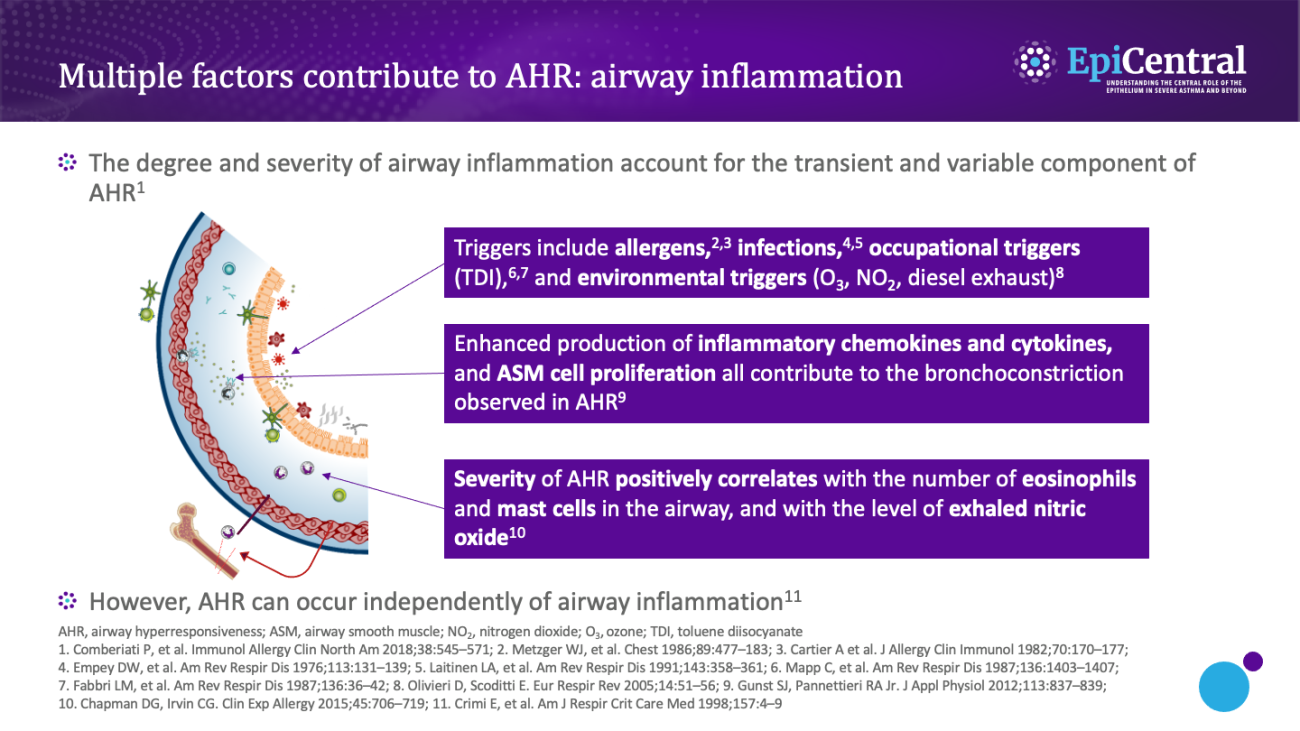
Inflammation forms part of the body’s natural defence mechanism, which under normal circumstances is beneficial.13 However, when the inflammation becomes chronic, it can lead to disease, as is the case with the chronic airway inflammation experienced by patients with asthma.13
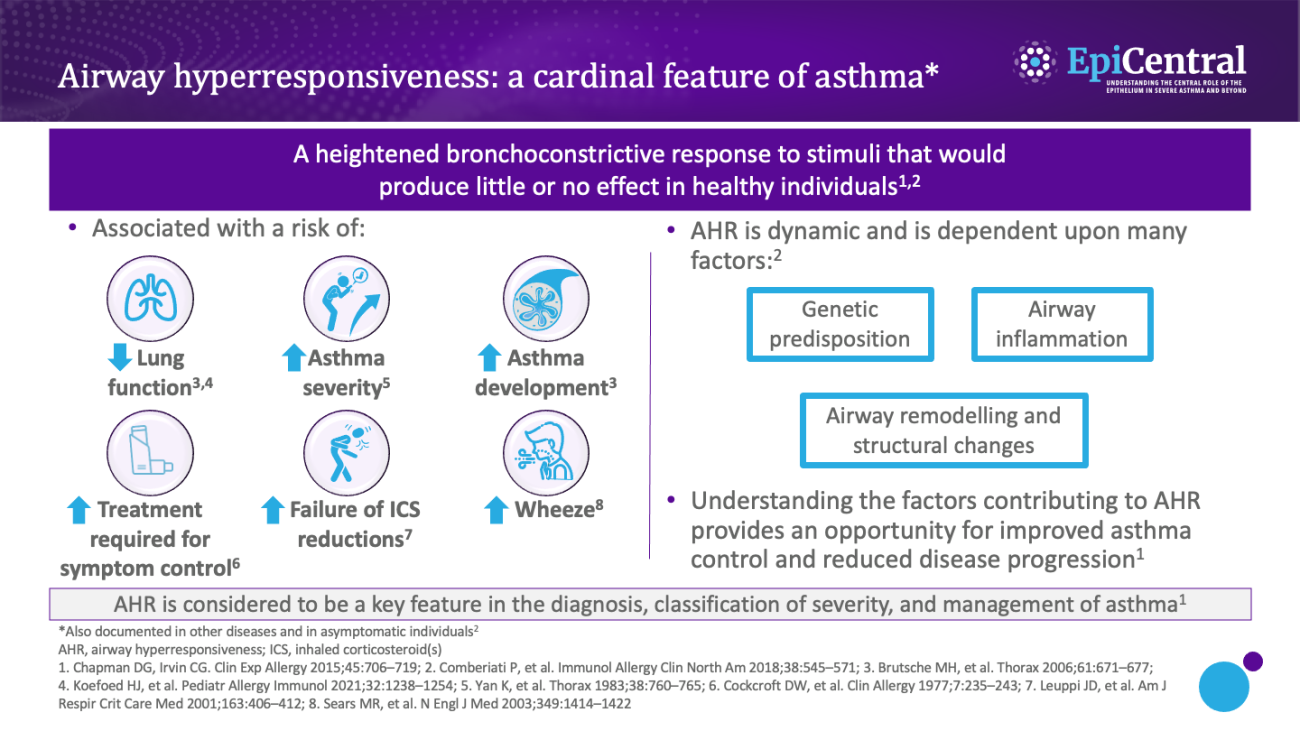
Airway inflammation can occur as a result of exposure to a range of factors, such as allergens, infections, occupational triggers, and environmental factors.1 As discussed in the Epithelial cytokines and the inflammatory cascade module, as part of the inflammatory pathway, epithelial cytokines are released from epithelial cells and induce the release of inflammatory cytokines (eg interleukin [IL]-4, IL-5, and IL-13) that drive inflammation and airway hyperresponsiveness.1,14 Airway inflammation is considered to be a transient, variable component of airway hyperresponsiveness,1 with the severity of airway hyperresponsiveness being associated with the number of inflammatory cells (including eosinophils and mast cells) in the airway.3
Mast cells play a critical role in airway inflammation and hyperresponsiveness; infiltration of mast cells into the ASM and the resultant secretion of proinflammatory and profibrotic mediators are thought to play a predominant role in the cellular and structural changes associated with airway hyperresponsiveness.6,15–17 Mast cells from human lungs have been shown, in vitro and in situ, to be in a continuously 'activated' state when cocultured with human ASM cells, with evidence of ongoing degranulation and expression of T2 cytokines.18